Our Quality Credentials
At Gracon Manufacturing, we are committed to delivering value to our customers each time they interact with our team. Quality shapes the foundation of this endeavor and is the cornerstone upon which Gracon Manufacturing builds lasting relationships.
We strive for the highest standard of quality that meets or exceeds expectations and requirements. We commit to continually improving our processes to maintain the effectiveness of our quality management system and remain our customers’ supplier of choice.

Quality Management System
certified to ISO 9001:2015 and ISO 13485:2016 standards by Platinum Registration, Inc.
TRAINING
Our Training Never Stops
Every employee at Gracon Manufacturing is involved in the success of our quality management system and trained to understand the importance and role they play in delivering quality to our customers and their products. Our process of continual improvement includes regular in-house and outsourced training to improve our knowledge and skillset and be a better partner to our customers.
Although Gracon Manufacturing is not regulated to 21 CFR 820 standards, we understand that many of our customers are. Our team is trained to understand your regulatory requirements and help you stay in compliance.

Our Facility
Built with Quality in Mind
Manufacturing medical components require expertise and a high level of process control. One way we have supported this effort is through the design of our facility, cleanroom, and equipment. Achieving the highest quality standards and meeting customer requirements was at the forefront of our past and future design decisions.

World class facilities
Cleanroom
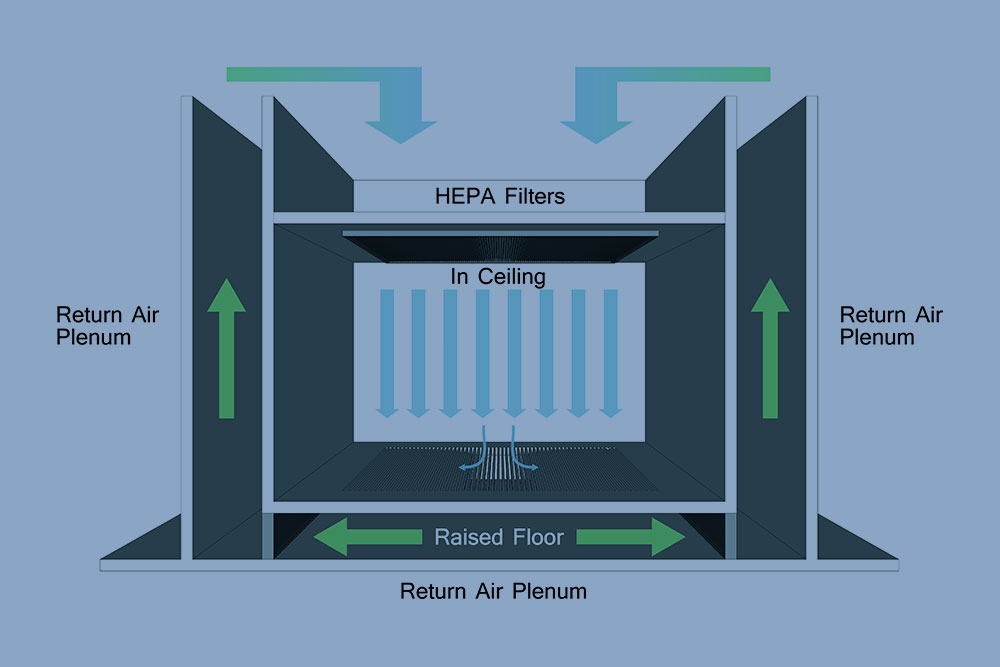
Our ISO 7 cleanroom was designed to accommodate multiple injection molding machines and automation while reducing the risk of contaminates. The space is equipped with anti-microbial flooring, 24 HEPA filters, and stainless-steel furniture able to withstand stringent cleaning and regular disinfecting required by our SOPs.
CONSISTANT QUALITY
White Room
Our white room neighbors our cleanroom and is used for assembly and other secondary operations. We follow the same standards and cleaning protocols as our cleanroom space, and the proximity allows for a seamless transfer from the cleanroom production area.


RISK MITIGATION
Chiller and Closed-Loop Monitored Water Treatment
Water plays a vital role in the injection molding process and adds potential contamination risk. Our state-of-the-art chiller system provides a closed-loop system to prevent contamination. The water used in the system is tested regularly and treated as needed to prevent damage to our equipment or your molds.
Documented In-process Inspections and Validation
- First Article Inspection (FAI)
- IQ (Installation Qualification)
- OQ (Operational Qualification)
- PQ (Performance Qualification)
- FAIRs (First Article Inspection Reports)
- Capability Studies
- Full DOEs (Design of Experiments)
- PPAPs (Product Part Approval Process)
- Complete Supply Chain Management
- Manufacturing and Tool Transfer